
EMA Marketing Authorization of New Drugs in December 2024
Shots:
-
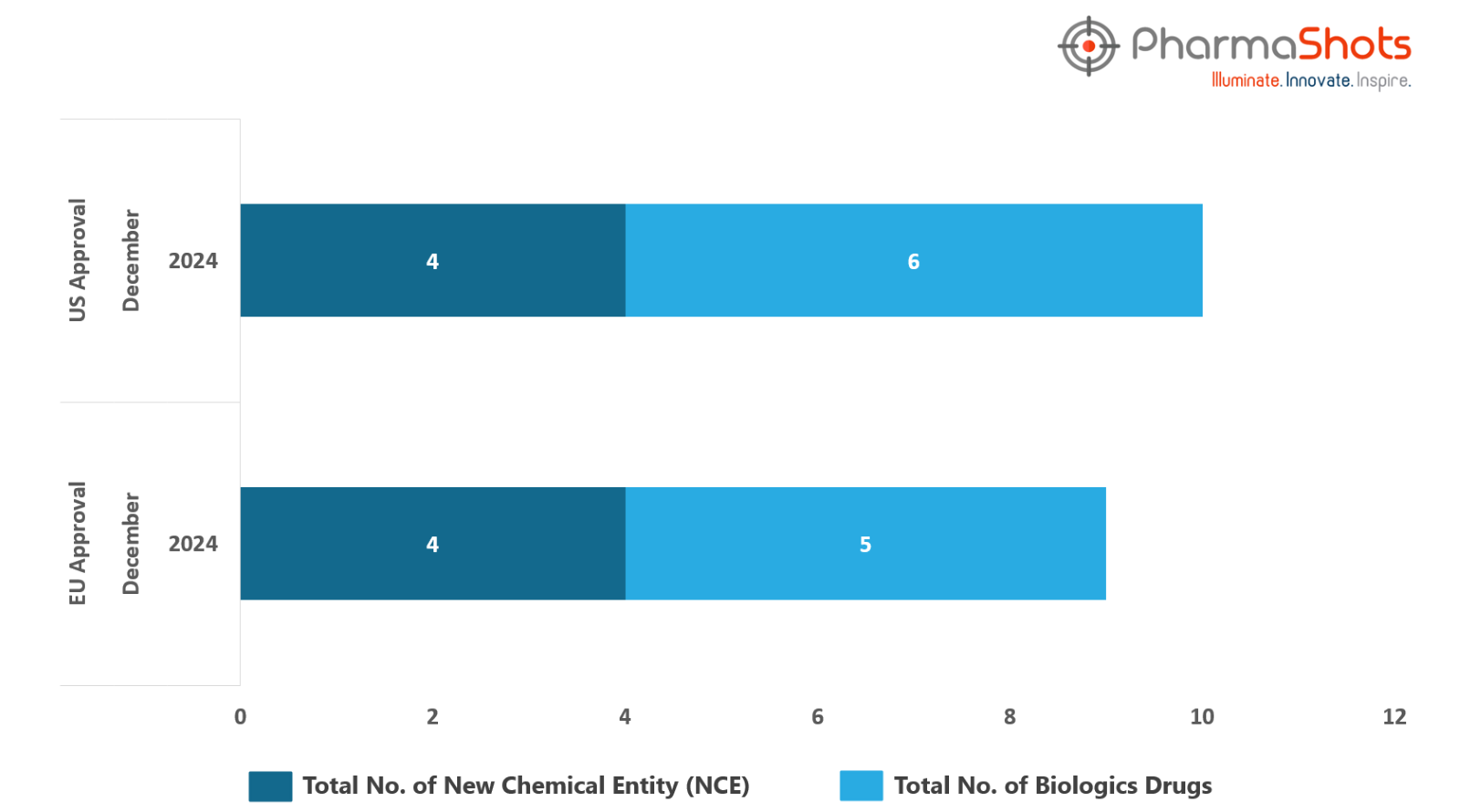
The EMA’s CHMP has granted positive opinions to 5 Biologics and 4 New Chemical Entities in December 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Galderma’s Nemluvio to treat Prurigo Nodularis and Atopic Dermatitis
-
PharmaShots has compiled a list of 9 drugs that have been granted positive opinions and approvals by the EMA’s CHMP & EC, respectively
Product Name: Beyonttra
Active ingredient: Acoramidis
Company: BridgeBio & Bayer
Date: Dec 12, 2024
Disease: Transthyretin Amyloidosis Cardiomyopathy
Shots:
-
The CHMP has recommended Beyonttra to treat wild-type or variant transthyretin amyloidosis cardiomyopathy (ATTR-CM) in adults, expected to launch in H1’25
-
Opinion was based on a P-III (ATTRibute-CM) study of Beyonttra (BID, oral) vs PBO in ATTR-CM patients. It achieved its 1EP, showing a 42% reduced composite ACM & recurrent CVH events & a 50% reduced cumulative frequency of CVH events at 30mos.
-
Acoramidis (TTR stabilizer) was developed by BridgeBio & Bayer. BridgeBio has US marketing rights for the drug, while Bayer holds them in the EU
2. Gilead Receives CHMP's Positive Opinion of Seladelpar to Treat Primary Biliary Cholangitis (PBC)
Product Name: Seladelpar
Active ingredient: Seladelpar
Company: Gilead
Date: Dec 12, 2024
Disease: Primary Biliary Cholangitis
Shots:
-
The CHMP has recommended Seladelpar + ursodeoxycholic acid (UDCA) to treat PBC in those with inadequate response or as monotx. in those intolerants to UDCA. The EC’s decision is expected in Q1’25
-
Opinion was based on a P-III (RESPONSE) study assessing seladelpar (10mg, QD, oral) vs PBO in PBC patients (n=193). It showed composite biochemical response in 62% vs 20% & ALP normalization of 25% at 12mos. (1EP) plus pruritus reduction of 3.2 vs 1.7 points at 6mos. (2EP)
-
Gilead is also pursuing regulatory approval for seladelpar in regions beyond the EU & under review by the UK MHRA. It is a PPAR- δ agonist that blocks bile acid synthesis to treat PBC
Product Name: Rytelo
Active ingredient: Imetelstat
Company: Geron
Date: Dec 12, 2024
Disease: Transfusion-Dependent Anemia due to Lower-Risk MDS
Shots:
-
The CHMP has recommended Rytelo to treat TD anemia in adults with very low, low, or intermediate risk non-del(5q) MDS, unresponsive or ineligible for erythropoietin-based therapy, with the EC’s decision expected in the coming months
-
Opinion was based on P-III (IMerge) study of Rytelo vs PBO that showed reduced need for RBC transfusions within the first 24wks., with common side effects of thrombocytopenia, leukopenia, neutropenia, elevated liver enzymes (AST, ALT, ALP), asthenia & headache
-
Rytelo is an oligonucleotide that inhibits uncontrolled cell division caused by over-expression of telomerase enzyme in LR-MDS; available as 47/188mg, IV infusion post approval
Product Name: Nemluvio
Active ingredient: Nemolizumab
Company: Galderma
Date: Dec 12, 2024
Disease: Prurigo Nodularis and Atopic Dermatitis
Shots:
-
The CHMP's recommendation of Nemluvio (SC) for mod. to sev. atopic dermatitis (≥12yrs.) & prurigo nodularis in eligible ones for systemic therapy was based on P-III ARCADIA & OLYMPIA studies, respectively. Reviews are ongoing in Australia, Singapore, Switzerland, Canada, Brazil & South Korea, with more filings underway
-
ARCADIA 1 & 2 studies assessed Nemluvio (Q4W) + TCS ± TCI vs PBO in atopic dermatitis patients (n=1,728) for 16 & 48wks., respectively. It showed improved co-1EPs & 2EPs as well as itch relief at wk.1
-
OLYMPIA1 & 2 trials assessed Nemluvio (Q4W) vs PBO in PN patients (n=560) for 16 & 24wks., respectively. It achieved its 1 & 2EPs, depicting improved itch & skin nodules at wk.16, with fast itch reductions at wk.4
Product Name: Andembry
Active ingredient: Garadacimab
Company: CSL Behring
Date: Dec 12, 2024
Disease: Hereditary Angioedema
Shots:
-
The CHMP has recommended Andembry as a prophylactic treatment of HAE in patients (≥12 yrs.), with the EC’s decision anticipated in Q1’25, based on P-III (VANGUARD) trial & ongoing OLE study
-
The P-III study (full results published in The Lancet) of Andembry vs PBO met its 1EP, showing attack-free status in 62% while reducing the median HAE attacks to 0 & mean HAE attacks/month by 86.5%. OLE study depicted sustained attack reduction & favorable long-term safety (primary results published in Allergy)
-
Garadacimab (QM) targets activated factor XII, responsible for attacks of swelling in HAE patients, to inhibit the HAE cascade thereby preventing attacks
6. Arcturus and Meiji Seika Pharma Report the EMA’s Positive Opinion of Kostaive for COVID-19
Product Name: Kostaive
Active ingredient: Zepomeran
Company: Arcturus Therapeutics and Meiji Pharma
Date: Dec 12, 2024
Disease: COVID-19
Shots:
-
The EMA’s CHMP has granted positive opinion to Kostaive (zepomeran) for active immunization to prevent COVID-19 in subjects of age ≥18yrs.
-
The EC’s decision on marketing authorization will follow subsequently
-
Kostaive is a self-amplifying mRNA vaccine that codes for the SARS-CoV-2 spike protein to provide active immunization against COVID-19
Product Name: Kavigale
Active ingredient: Sipavibart
Company: AstraZeneca & RQ Biotechnology
Date: Dec 12, 2024
Disease: COVID-19
Shots:
-
The EMA’s CHMP has granted positive opinion to Kavigale for the prevention of COVID-19 in immunocompromised individuals (≥12yrs.). It was reviewed under the EMA’s accelerated pathway
-
Kavigale consists of an antiviral human IgG1 mAb, sipavibart, as its API that offers passive protection against SARS-CoV-2 by targeting the spike protein's receptor-binding domain
-
Sipavibart was discovered by RQ Bio and The University of Oxford. It was licensed to AstraZeneca as per an agreement signed in May 2022
Product Name: Welireg
Active ingredient: Belzutifan
Company: Merck
Date: Dec 14, 2024
Disease: Von Hippel-Lindau & Renal Cell Carcinoma
Shots:
-
The CHMP has recommended conditional approval of Welireg for VHL-related localized RCC, CNS hemangioblastomas or pNET unsuitable for localized procedures and advanced ccRCC post PD-1/PD-L1 inhibitors or VEGF therapies progression. EC’s decision is anticipated in Q1’25
-
Opinion for VHL-related tumors was based on LITESPARK-004 study (n=61), showing ORR of 49% (all PRs), with 56% maintaining response (RCC); 63% (CR: 4%, PR: 58%), with 73% maintaining response (CNS hemangioblastomas) & 83% (CR: 17%, PR: 67%), with 50% maintaining response for ≥12mos. (pNET); mDoR not reached
-
Opinion for ccRCC was based on P-III (LITESPARK-005) study (n=746), depicting reduced disease progression or death risk by 25% vs everolimus, with mPFS of 5.6mos., ORR of 22% (CR: 3%, PR: 19%) vs 4% (PR: 4%)
Product Name: Rybrevant + Lazcluze
Active ingredient: Amivantamab + Lazertinib
Company: Johnson & Johnson
Date: Dec 30, 2024
Disease: EGFR-Mutated NSCLC
Shots:
-
The EC has approved Rybrevant + Lazcluze as a 1L treatment of advanced NSCLC harboring EGFR exon 19 deletions (ex19del) or exon 21 L858R substitution mutations. The EC’s decision of lazertinib for the corresponding combination regimen is pending (was granted CHMP’s recommendation last month)
-
Approval was based on P-III (MARIPOSA) trial assessing Rybrevant + Lazcluze vs osimertinib or Lazcluze alone as a 1L treatment of locally advanced or metastatic NSCLC patients (n=1,074) with EGFR ex19del or L858R substitution mutations
-
Study achieved its 1EP of PFS, showing reduced risk of disease progression or death by 30% vs osimertinib, with mPFS of 23.7 vs 16.6mos. (at 22mos. median follow-up) & mDoR of 25.8 vs 16.8mos. (9mos. improvement)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in November 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














